(lat. lansoprazol, eng. lansoprazole) - a drug that lowers the acidity of the stomach, a proton pump inhibitor. Previously sometimes called Lansoprazole.
Lansoprazole - chemical
Chemical compound : 2 - [[methyl] sulfinyl] benzimidazole. Empirical formula C 16 H 14 F 3 N 3 O 2 S.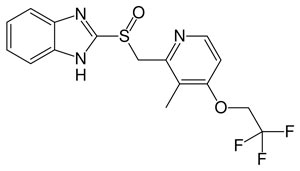
Lansoprazole is a substituted benzimidazole derivative. Odorless whitish-brownish granular powder. Lansoprazole is moderately soluble in ethyl alcohol, insoluble in hexane and water. Lansoprazole is resistant to light. At 25 ° C, lansoprazole decomposes in an acidic medium at = 5.0 with T½ = 0.5 hours and at T½ = 18 hours in a neutral medium (at pH = 7.0).
Lansoprazole - medicine
Lansoprazole is the international non-proprietary name (INN) of the drug. According to the pharmacological index, it belongs to the group "Proton pump inhibitors". According to ATX - to the group "Proton pump inhibitors" and has the code A02BC03.It is also the trade name of a number of drugs.
Indications for the use of lansoprazole

- nonulcer dyspepsia
- stomach and / or duodenal ulcer,
- erosive and ulcerative esophagitis
- reflux esophagitis
- Zollinger-Ellison syndrome,
- eradication Helicobacter pylori as part of combination therapy (monotherapy with lansoprazole is not used for eradication).
Lansoprazole route and dosage
Lansoprazole is taken by mouth, preferably in the morning.- non-ulcer dyspepsia: 15-30 mg daily for 2-4 weeks
- duodenal ulcer: 30 mg daily for 2-4 weeks
- stomach ulcer: 30-60 mg daily for 4-8 weeks
- erosive-ulcerative esophagitis: 30-60 mg per day for 4-8 weeks
- reflux esophagitis: 30 mg daily - 4 weeks
- Zollinger-Ellison syndrome: in an individually adjusted dose that provides a level of basal acid production below 10 mmol / hour
- eradication Helicobacter pylori: 30 mg 2 times a day only in combination with antibiotics; the duration of the course depends on the scheme (see. Diagnostic and Treatment Standards for Acid Dependent and Associated Helicobacter pylori diseases, which detail the various eradication schemes).
 When prescribing lansoprazole, it is necessary to take into account its following features. The absorption rate and bioavailability of lansoprazole is twice as high when the drug is taken in the morning as compared to the evening. The bioavailability of lansoprazole is reduced when the drug is taken simultaneously with food and antacids. With an equal daily dose, taking the drug twice a day is accompanied by a more pronounced antisecretory effect compared to a single dose. Lansoprazole has a more pronounced antisecretory effect than H2 blockers. Unlike other PPIs, lansoprazole in high concentration is excreted in milk (Alekseenko S.A.).
When prescribing lansoprazole, it is necessary to take into account its following features. The absorption rate and bioavailability of lansoprazole is twice as high when the drug is taken in the morning as compared to the evening. The bioavailability of lansoprazole is reduced when the drug is taken simultaneously with food and antacids. With an equal daily dose, taking the drug twice a day is accompanied by a more pronounced antisecretory effect compared to a single dose. Lansoprazole has a more pronounced antisecretory effect than H2 blockers. Unlike other PPIs, lansoprazole in high concentration is excreted in milk (Alekseenko S.A.). On the Russian market, lansoprazole is represented by generics. Due to possible differences in the quality of drugs, an objective assessment of their clinical efficacy is often necessary. Currently, 24-hour monitoring of intragastric pH is an objective and affordable method for testing antisecretory drugs in clinical practice (Alekseenko S.A.).
Comparison of lansoprazole with other proton pump blockers
 There are different points of view among gastroenterologists regarding the comparative effectiveness of specific types of proton pump inhibitors. Some of them argue that, despite some differences that exist between PPIs, today there are no convincing data that allow us to talk about the greater effectiveness of any PPI in comparison with the rest (Vasiliev Yu.V. et al.). Also, a number of gastroenterologists believe that during the eradication of Hp, the type of PPI used in combination with antibiotics does not matter (Nikonov E.K., Alekseenko S.A.). Others write that, for example, esomeprazole is fundamentally different from the other four PPIs: omeprazole, pantoprazole, lansoprazole and rabeprazole (Lapina T.L., Demyanenko D. and others).
There are different points of view among gastroenterologists regarding the comparative effectiveness of specific types of proton pump inhibitors. Some of them argue that, despite some differences that exist between PPIs, today there are no convincing data that allow us to talk about the greater effectiveness of any PPI in comparison with the rest (Vasiliev Yu.V. et al.). Also, a number of gastroenterologists believe that during the eradication of Hp, the type of PPI used in combination with antibiotics does not matter (Nikonov E.K., Alekseenko S.A.). Others write that, for example, esomeprazole is fundamentally different from the other four PPIs: omeprazole, pantoprazole, lansoprazole and rabeprazole (Lapina T.L., Demyanenko D. and others). For maintenance therapy of GERD, a daily intake of 15 mg of lansoprazole is recommended, which is comparable in effectiveness to 20 mg of omeprazole, but inferior to esomeprazole at a dose of 20 mg. The cost of generics of lansoprazole and omeprazole is much lower than that of esomeprazole and rabeprazole, which is of no small importance for the patient and often determines the choice of a drug based on financial capabilities, especially for long-term use (Alekseenko S.A.).
Professional medical articles regarding the use of lansoprazole
- Alekseenko S.A. Lansoprazole is the drug of choice in the treatment of acid-related diseases // Farmateka. - 2007. - No. 13 (147). - with. 19-22.
- Astakhov A.L. Lansoprazole: optimization of antiulcer therapy // Medical newspaper "Health of Ukraine"
- Zakharova N.V. Lansoprazole: features of the clinical pharmacology of PPIs // Clinical gastroenterology and hepatology. - 2008. - volume 1. - no. 3. - p. 205-211.
- Osipenko M.F., Bikbulatova E.A., Shakalite Yu.D. The effectiveness of Lanzoptol for relieving heartburn in patients with NERD // Experimental and Clinical Gastroenterology. - 2009. - No. 4.
- Kurilovich S.A., Chernosheikina L.E., Shlykova L.G. Lansoprazole and gastric blockade: are there any differences among generics? Clinical perspectives of gastroenterology, hepatology. No. 3, 2007.
Pharmacological action of lansoprazole
Lansoprazole is an anti-ulcer drug that reduces gastric acidity. Lansoprazole blocks the final stage of hydrochloric acid formation. In the tubules of the parietal cells of the stomach, it is transformed into an active form - sulfonamide, which irreversibly interacts with the SH-groups of H + / K + -ATPase (proton pump). Lansoprazole reduces basal and stimulated secretion and secretion volume. The rate and degree of inhibition of the secretion of hydrochloric acid by lansoprazole are dose-dependent: after taking 15 and 30 mg of lansoprozole, the acidity of the stomach begins to decrease after 1-2 and 2-3 hours, and the secretion decreases to 80-97%, respectively. Recovery of H + / K + -ATPase activity occurs with a half-period of 30–48 hours. The average daily value of gastric acidity rises to 2.9 pH (the percentage of time for maintaining pH> 3 is 47.6). After stopping lansoprazole, the acid level remains below 50% basal for 39 hours, "acid rebound" is not observed. In patients with Zollinger-Ellison syndrome, lansoprazole works longer. Inhibits the production of pepsin (increases the level of pepsinogen in serum). Has a gastroprotective effect: increased oxygenation of the mucous membrane, increased secretion of bicarbonates. Lansoprazole inhibits the growth of Helicobacter pylori, promotes the formation of specific IgA to these bacteria in the mucous membrane, increases the anti-Helicobacter activity of other drugs. Lansoprazole reduces blood flow in the antrum, pylorus and duodenal bulb by an average of 17%, inhibits the motor-evacuation function of the stomach. The suppression of secretion is accompanied by an increase in the number of nitrosobacteria and an increase in the concentration of nitrates in gastric secretions. Lansoprazole increases the serum gastrin concentration by 50-100% (gastrin level reaches a plateau after two months of treatment and returns to baseline values after the end of the course of treatment). Lansoprazole provides faster healing and amelioration of symptoms in duodenal ulcers (85% of duodenal ulcers heal after 4 weeks of treatment at a dose of 30 mg per day). Lansoprazole is effective in the treatment of H2-blocker-resistant gastric and duodenal ulcers. The recurrence rate of peptic ulcers after lansoprazole treatment is 55–62%. With reflux esophagitis, a complete cure by the end of the 8th week of admission (30 mg per day) is observed in 88.7% of patients.
Lansoprazole reduces basal and stimulated secretion and secretion volume. The rate and degree of inhibition of the secretion of hydrochloric acid by lansoprazole are dose-dependent: after taking 15 and 30 mg of lansoprozole, the acidity of the stomach begins to decrease after 1-2 and 2-3 hours, and the secretion decreases to 80-97%, respectively. Recovery of H + / K + -ATPase activity occurs with a half-period of 30–48 hours. The average daily value of gastric acidity rises to 2.9 pH (the percentage of time for maintaining pH> 3 is 47.6). After stopping lansoprazole, the acid level remains below 50% basal for 39 hours, "acid rebound" is not observed. In patients with Zollinger-Ellison syndrome, lansoprazole works longer. Inhibits the production of pepsin (increases the level of pepsinogen in serum). Has a gastroprotective effect: increased oxygenation of the mucous membrane, increased secretion of bicarbonates. Lansoprazole inhibits the growth of Helicobacter pylori, promotes the formation of specific IgA to these bacteria in the mucous membrane, increases the anti-Helicobacter activity of other drugs. Lansoprazole reduces blood flow in the antrum, pylorus and duodenal bulb by an average of 17%, inhibits the motor-evacuation function of the stomach. The suppression of secretion is accompanied by an increase in the number of nitrosobacteria and an increase in the concentration of nitrates in gastric secretions. Lansoprazole increases the serum gastrin concentration by 50-100% (gastrin level reaches a plateau after two months of treatment and returns to baseline values after the end of the course of treatment). Lansoprazole provides faster healing and amelioration of symptoms in duodenal ulcers (85% of duodenal ulcers heal after 4 weeks of treatment at a dose of 30 mg per day). Lansoprazole is effective in the treatment of H2-blocker-resistant gastric and duodenal ulcers. The recurrence rate of peptic ulcers after lansoprazole treatment is 55–62%. With reflux esophagitis, a complete cure by the end of the 8th week of admission (30 mg per day) is observed in 88.7% of patients.
The simultaneous use of lansoprazole and ceftriaxone (an antibacterial drug from the cephalosporin group) in some patients leads to a significant lengthening of the QT interval, which, in turn, significantly increases the risk of bidirectional ventricular tachycardia, which can lead to sudden death (Lorberbaum T. et al. Coupling Data Mining and Laboratory Experiments to Discover Drug Interactions Causing QT Prolongation. J Am Coll Cardiol 2016; 68: 1756-1764).
Lansoprazole overdose
A single dose of 600 mg of lansoprazole is not accompanied by clinical manifestations of an overdose. In case of an overdose of lansoprazole, observation, supportive and symptomatic therapy is recommended. Hemodialysis is ineffective.Precautions for lansoprazole treatment
To exclude malignant neoplasms before and after lansoprazole therapy, gastroduodenoscopy is necessary, since lansoprazole can mask symptoms and delay the diagnosis. Lansoprazole is used with caution in patients with reduced liver function and in elderly patients. Treatment begins with half doses, gradually increasing them to the recommended dose, but not more than 30 mg per day. When used simultaneously with antacids, they are taken one hour before or 1-2 hours after lansoprazole.Use of lansoprazole during pregnancy and breastfeeding
Lansoprazole is contraindicated in the first trimester. In the II and III trimesters, the use of lansoprazole is possible if the expected benefit of therapy outweighs the potential risk to the fetus. The FDA risk category for the fetus when taking a pregnant lansoprazole is B (animal studies have not identified the risks of a negative effect of lansoprazole on the fetus, there have been no proper studies in pregnant women). Breastfeeding should be discontinued during lansoprazole treatment.Trade names of drugs with the active ingredient lansoprazole
 Medicines with the active substance lansoprazole, registered in Russia lancerol (JSC Kievmedpreparat, Ukraine).
Medicines with the active substance lansoprazole, registered in Russia lancerol (JSC Kievmedpreparat, Ukraine). Prescribing Instructions for Certain Lansoprazole Products
- Instructions for the medical use of the drug Lanzabel (enteric capsules, 30 mg), (pdf, download).
- Instructions for the medical use of the drug Lanzoptol (capsules, 30 mg), (pdf, download)
- Instruction (medical guide) for US patients from the manufacturer (in English, pdf): "Prevacid (lansoprazole) Delayed-Release Capsules and Prevacid SoluTab (lansoprazole) Delayed-Release Orally Disintegrating Tablets"
Lansoprazole is the most popular gastrointestinal drug in the US
 Lansoprazole is the leading prescription drug in the United States for the treatment of diseases of the digestive system. In 2004, about 20 million prescriptions for lansoprozole were written in the United States, just over $ 3 billion was spent on the purchase of prescription lansoprazole in 2004, that is, more than a quarter of all funds spent on the purchase of prescription drugs for the treatment of the gastrointestinal tract.
Lansoprazole is the leading prescription drug in the United States for the treatment of diseases of the digestive system. In 2004, about 20 million prescriptions for lansoprozole were written in the United States, just over $ 3 billion was spent on the purchase of prescription lansoprazole in 2004, that is, more than a quarter of all funds spent on the purchase of prescription drugs for the treatment of the gastrointestinal tract.Branded a prescription drug with the active ingredient lansoprazole, registered in the USA and several other countries, but not registered in the Russian Federation - Prevacid. Its over-the-counter version, which contains a reduced amount of lansoprazole - 15 mg, is approved by the FDA for sale in the United States as an over-the-counter medicine for treating heartburn - Prevacid 24HR. The manufacturer of the prevacid is Takeda Pharmaceutical Co Ltd (Japan). ...
Lansoprazole has contraindications, side effects and application features, consultation with a specialist is necessary.

